Titration Hcl Mit Naoh . learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Ml of water at 25.0°c. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. Includes kit list and safety instructions. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution?
from www.numerade.com
learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Ml of water at 25.0°c. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. Includes kit list and safety instructions. a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid.
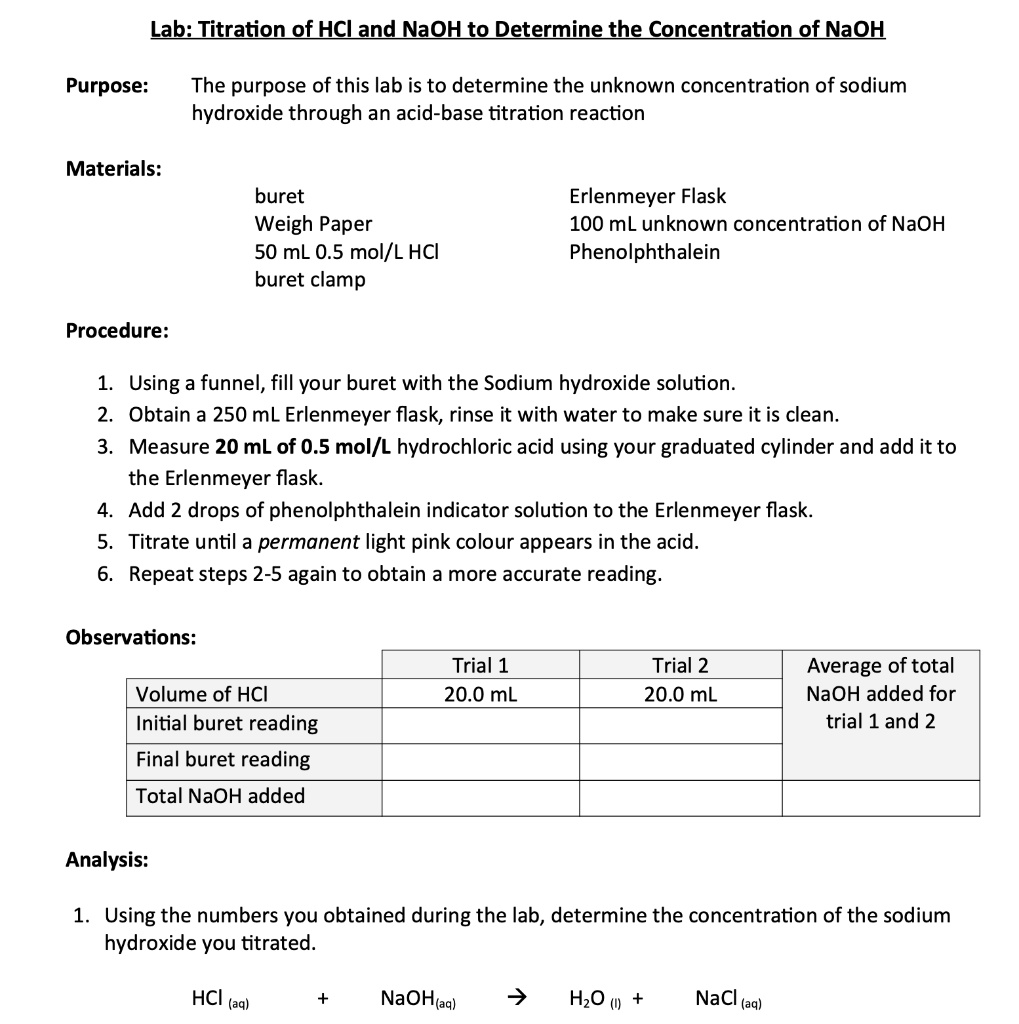
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH
Titration Hcl Mit Naoh learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. Includes kit list and safety instructions. Ml of water at 25.0°c. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution?
From mungfali.com
HCl NaOH Titration Titration Hcl Mit Naoh learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Ml of water at 25.0°c. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00. Titration Hcl Mit Naoh.
From www.studypool.com
SOLUTION Titration with hcl and naoh Studypool Titration Hcl Mit Naoh learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Ml of water at 25.0°c. use this class practical to explore titration, producing the salt sodium. Titration Hcl Mit Naoh.
From mungfali.com
HCl NaOH Titration Titration Hcl Mit Naoh learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Ml of water at 25.0°c. Includes kit list and safety instructions. a 0.10 m naoh solution. Titration Hcl Mit Naoh.
From mungfali.com
HCl NaOH Titration Titration Hcl Mit Naoh Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Ml of water at 25.0°c. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. learn how to titrate sodium hydroxide with hydrochloric acid using. Titration Hcl Mit Naoh.
From jeanius123.weebly.com
Chemistry Titration of HCl using NaOH Titration Hcl Mit Naoh learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Includes kit list and safety instructions. Ml of water at 25.0°c. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? learn how to determine the. Titration Hcl Mit Naoh.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Titration Hcl Mit Naoh Includes kit list and safety instructions. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. Ml of water at 25.0°c. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange. Titration Hcl Mit Naoh.
From www.youtube.com
Titration calculation 1 NaOH Vs HCl YouTube Titration Hcl Mit Naoh Ml of water at 25.0°c. Includes kit list and safety instructions. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. learn how to determine the. Titration Hcl Mit Naoh.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Hcl Mit Naoh Includes kit list and safety instructions. a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. Ml of water at 25.0°c. use this class practical to explore titration, producing. Titration Hcl Mit Naoh.
From studymoose.com
Titration with HCl and NaOH Free Essay Example Titration Hcl Mit Naoh learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Ml of water at 25.0°c. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. . Titration Hcl Mit Naoh.
From www.youtube.com
Titration of HCl with NaOH YouTube Titration Hcl Mit Naoh Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. Includes kit list and safety instructions. learn how to titrate sodium. Titration Hcl Mit Naoh.
From www.youtube.com
pH Titration HCl + NaOH mit Teacher's Helper AK Kappenberg Chemie Titration Hcl Mit Naoh Ml of water at 25.0°c. Includes kit list and safety instructions. learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? use this class practical to. Titration Hcl Mit Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Titration Hcl Mit Naoh a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? learn how to determine the quantitative composition of a solution that. Titration Hcl Mit Naoh.
From www.youtube.com
NaOH vs HCl Titration using Phenolphthalein Class 11 Chemistry Lab Titration Hcl Mit Naoh learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Includes kit list and safety instructions. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? use this class practical to explore titration, producing the salt. Titration Hcl Mit Naoh.
From mungfali.com
HCl NaOH Titration Titration Hcl Mit Naoh learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. Example \(\pageindex{3}\) what is the ph. Titration Hcl Mit Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Hcl Mit Naoh learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. Ml of water at 25.0°c. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange. Titration Hcl Mit Naoh.
From www.researchgate.net
Titration of HCl (0.1M) against NaOH (0.1M) Download Scientific Diagram Titration Hcl Mit Naoh learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. learn how to titrate sodium hydroxide with hydrochloric acid using methyl orange indicator and how to deal. Includes kit list and safety instructions.. Titration Hcl Mit Naoh.
From mungfali.com
HCl NaOH Titration Titration Hcl Mit Naoh use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Example \(\pageindex{3}\) what is the ph when 48.00 ml of 0.100 m naoh solution have been added to 50.00 ml of 0.100 m hcl solution? Ml of water at 25.0°c. learn how to determine. Titration Hcl Mit Naoh.
From www.alamy.de
Titration mit HCl NaOH und Phenolphthalein hinzugefügt wird. Erste in Titration Hcl Mit Naoh Ml of water at 25.0°c. Includes kit list and safety instructions. a 0.10 m naoh solution is used to titrate a 0.295 g sample of an unknown acid that was dissolved in 40. learn how to determine the quantitative composition of a solution that contains a monoprotic strong acid. use this class practical to explore titration, producing. Titration Hcl Mit Naoh.